NEWS, EVENTS & BLOG
BLOG

The Best SBCs for Medical Equipment Applications
Press Office, VersaLogic Corporation, 05/13/21
Manufacturers of medical equipment aren’t only focused on creating innovative solutions to medical problems. Development of medical devices and systems poses additional challenges including:
- High cost of product design and development: The primary cost-driver of development is the lengthy time between the initial concept and product release. One way of reducing this cost is to use as many off-the-shelf building blocks as possible.
- Regulatory compliance and certification: Medical equipment manufacturers are operating in a regulated industry. The regulatory authority in the US is the US Food and Drug Administration (FDA). To gain approval a company must prove that a device is safe and effective. A recent article estimated that it takes, on average, between approximately six and eight months to gain approval for Class 2 and 3 medical devices.
- Security: Due to the proliferation of network-connected medical devices, ensuring data security is an increasingly important challenge.
- Product quality: Poor product quality can lead to patient injury or worse. Recalls can negatively impact a brand and bottom line. Ensuring product quality is extremely important for manufacturers of medical equipment.
 Medical equipment must be safe, reliable, long lifecycle, and be certified
Medical equipment must be safe, reliable, long lifecycle, and be certified
When looking for the best single board computer (SBC) it’s not enough just to select one that meets the required features. It’s critical to select one from a supplier that has experience of working with medical applications and can help meet the four challenges listed above.
Minimizing Development Cost and Time
The lowest development cost and quickest option to procure an SBC is to choose a commercial-off-the-shelf (COTS) industrial computer. In some cases, it might be necessary to tweak the COTS product to make it fit with a specific application. It’s therefore important to choose a supplier that has significant experience in adapting COTS products. VersaLogic calls this their Modified COTS (MCOTS) process.
Selecting a COTS or MCOTS board saves significant time and money compared with a medical equipment manufacturer designing their own SBC. If a truly ground-up new SBC is required, then having an established and experienced SBC supplier design and build a custom SBC is another option.
Whether using a COTS, MCOTS, or custom designed board, purchasing from an established SBC supplier also has ongoing cost savings. The supplier will bear the cost and effort of on-going supply chain management, including component end-of-life issues.
 The COTS BayCat SBC used in RF tissue remodeling system
The COTS BayCat SBC used in RF tissue remodeling system
Regulatory Compliance and Certification
An SBC supplier can contribute to solving quality and compliance issues through capabilities such as revision locking for a supplied product. This ensures that the product will continue to be supplied identically to when it was certified for use in a given system.
It is also important to have a track record and the ability to supply SBCs over the long term. Choosing an SBC supplier that has processes in place to provide long lifecycle products is critical to avoiding the headaches of re-design and re-certification. VersaLogic’s approach to product longevity includes:
- Selecting the right parts for a long-life design; in particular looking to use multi-source long-life components.
- Proactive supply chain management and close vendor relationships supporting early notification of component availability changes.
- Carrying a deep in-house stock of hard-to-get parts. Warehousing in inert gas environments when appropriate.
- Formal lifecycle extension programs.
- Providing at least 18 months’ notice for ending off-the-shelf availability. Plenty of time to set up a life extension program if needed.
There have even been cases where VersaLogic has resurrected obsolete products for customers that discover an unanticipated need to extend their product lifetime.
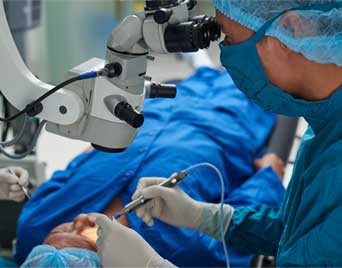
 An MCOTS version of the Tomcat used in eye surgery phacoemulsion systems since 2014
An MCOTS version of the Tomcat used in eye surgery phacoemulsion systems since 2014
Technology and Security
The SBC used in a system is a major contributor to security through technological means. Rather than repeating the details here, three previously posted blogs provide insights into important contributions that modern SBCs make to data security.
- Processor-based security measures
- The use of Trusted Platform Modules (TPM)
- Securing remotely connected systems
 An MCOTS version of the Bengal SBC is used in a blood apheresis system
An MCOTS version of the Bengal SBC is used in a blood apheresis system
Product Quality and Recall Rates
 An SBC can support product quality in a number of ways. The most important being strict quality processes.
An SBC can support product quality in a number of ways. The most important being strict quality processes.
ISO9001 is familiar to most SBC users. However, some suppliers, such as VersaLogic, go beyond and have been certified to AS9100. This directly addresses risk management as relates to product safety and counterfeit parts. This makes it relevant to any customer where minimizing risk to proper product functioning and end user safety is important. AS9100’s risk-tolerance is more stringent than the requirements within ISO 9001 with much more focus on prevention rather than corrective action.
 The low power Wi-Fi equipped Swordtail SBC is ideal for portable medical applications
The low power Wi-Fi equipped Swordtail SBC is ideal for portable medical applications
The Bottom Line
The best SBC for medical equipment is not just the one with the required features and performance. For medical applications, it must be supplied by a company with a history and understanding of what it takes to meet the non-technical requirements of:
- Minimizing development cost and timeline by supplying COTS or MCOTS products with the medical industry in mind
- Minimizing re-certifications by having a demonstrated track record of long product lifecycles
- Addressing system security issues
- Minimizing re-calls and maximizing MTBF by having processes and certifications in place to ensure high quality products
Need additional Information?
Want to know more about VersaLogic’s range of embedded products? Let’s start a conversation.
